Properties:
- Ethyl 2-Cyano-2-(Hydroxyimino)Acetate
- CAS 3849-21-6
- C5H6N2O3
- MW 142.11
- PKa 4,6 +/- 0.4
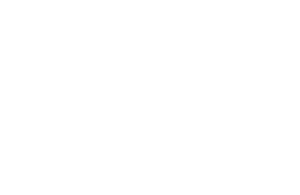


The most common strategy for the synthesis of amide bonds is the condensation of amines with carboxylic acids in the presence of carbodiimides, (EDC HCl, DIC, TBEC). Carbodiimides react with the carboxylic group to render the highly reactive O-acylisourea (see scheme). Due to its high reactivity, O-acylisourea decomposes rapidly and/or undergoes rearrangement to form the totally inactive N-acylurea or in the case of the carbamate protected α-amino acids, it evolves to oxazolone, which shows poor reactivity and provokes the loss of chiral integrity. To mitigate this inefficiency in the carbodiimide-mediated coupling, a coupling additive such as OxymaPure®, HOBt, HOPO, or others can be added to the coupling cocktail. Since the active species formed shows increased stability, has excellent acylation capabilities, and significantly minimizes the side-reactions associated with O-acylisourea.
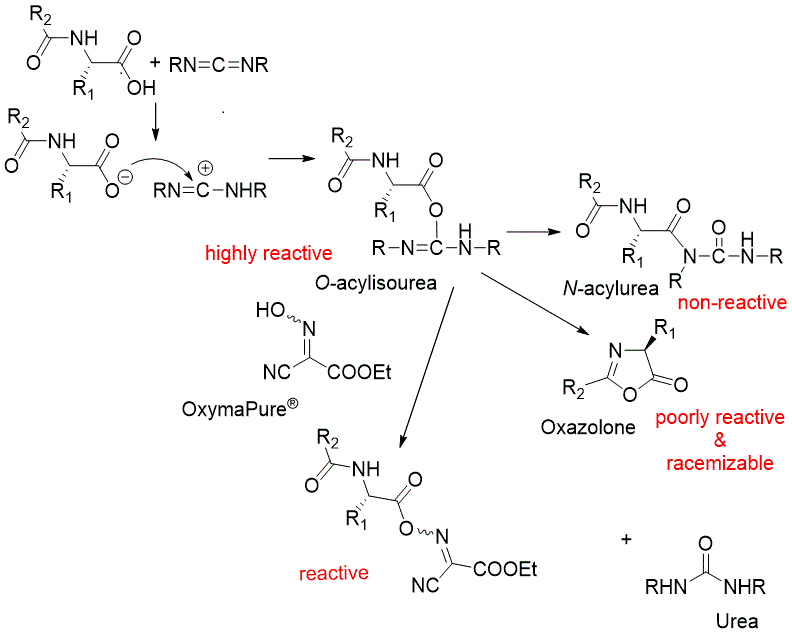
OxymaPure®, Ethyl 2-Cyano-2-(Hydroxyimino) Acetate an ideal additive used with carbodiimide coupling methodology,
for peptide/amide bond formation and it is the registered trademark of Luxembourg Bio Technologies Ltd.
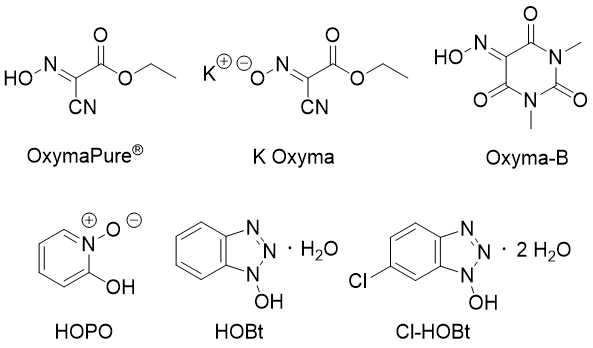
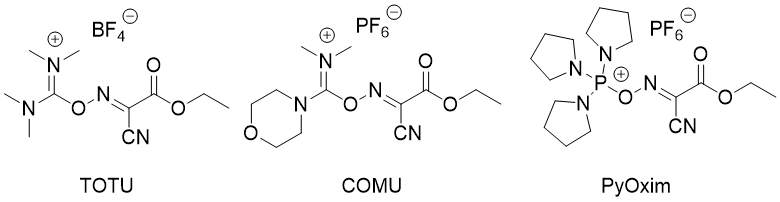
OxymaPure® is also the base for several stand-alone coupling reagents with superior performance when compared with similar reagents (15, 16).
OxymaPure® is also the base for several stand-alone coupling reagents with superior performance when compared with similar reagents (15, 16).
